Covaxin Who Approval Date / India Gets 2 Vaccines Against Coronavirus As Covaxin Covishield Get Final Nod By Dcgi
WHO an independent group of experts are scheduled to meet next week to carry out the riskbenefit assessment and come to a final decision whether to grant Emergency Use Listing. The latest news is the World Health Organization approval for the emergency use authorization EUA to Bharat Biotech-manufactured Covid-19 vaccine- Covaxin is likely to be delayed till.
Who S Emergency Use Approval To Covaxin Delayed Till October 5 India News India Tv
A decision on Bharat Biotechs submission seeking emergency use listing EUL for its Covaxin COVID-19 vaccine will be made in October the World Health Organisation has said.

Covaxin who approval date. However the World Health Organisations approval for the emergency use authorisation EUA to Covid-19 vaccine Covaxin is likely to be delayed as per international public health agency. The WHO has approved COVID-19 vaccines by Pfizer-BioNTech AstraZeneca Johnson and Johnson Moderna and Sinopharm till date. WHO panels four-day meeting begins today.
Collection of Covaxin who approval date The World Health Organisations approval for emergency use of Covaxin can come before the end of the month Dr VK Paul chairman of the National Expert Committee on. 1 day agoCBSE Term 1 Exam 2021. On September 29 WHO had updated the decision date for Covaxin EUL to October 2021 in its latest EUL guidance document for Covid-19 vaccines from the yet to be confirmed status.
Covaxin who approval date. The World Health Organisation WHO will take a call on granting Emergency Use Listing EUL status for Bharat Biotechs COVID-19 vaccine Covaxin next week the global health body said on Tuesday. As we know it recently has been searched by users around us perhaps one of you.
Collection of Covaxin who approval date The World Health Organisations approval for emergency use of Covaxin can come before the end of the month Dr VK Paul chairman of the National Expert Committee on. WHO decision on Covaxin nod extended to next week -. Covaxin decision date to be confirmed says WHO Premium A health worker preparing a dose from a vial of Covaxin a Covid-19 vaccine.
Covaxin decision date to be confirmed says WHO Premium A health worker preparing a dose from a vial of Covaxin a Covid-19 vaccine. The FDA has cited insufficient information on Covaxin to grant approval to its emergency use. The status of the assessment for Covaxin is ongoing.
WHO an independent group of experts are scheduled to meet next week to carry out the riskbenefit assessment and come to a final decision whether. As of date India is using three vaccines against COVID-19 in its immunization drive. Pfizer-BioNTech jab Astrazeneca vaccine developed by SK Bio and Serum Institute.
Read more about Hester Bio spurts after Covaxin gets emergency use approval for kids on Business Standard. In an update on its website the WHO which began rolling data on July 6 said the date for a decision on the jab is yet to be confirmed. The latest Status of COVID-19 vaccines.
The document mentions the status of Covaxins assessment by the WHO as ongoing and the decision date as October 2021. Covaxin for Children will be in two doses. Bharat Biotech had submitted EOI Expression of Interest on 19 April for its COVID-19 vaccine.
A decision on Bharat Biotechs submission seeking emergency use listing EUL for its Covaxin COVID-19 vaccine will be made in October the World Health Organisation has said. Collection of Covaxin who approval Developed by Bharat Biotech in partnership with the National Institute of Virology and the Indian Council of Medical Research ICMR Covaxin was approved for emergency use on January 3. These include two made-in-India vaccines - Serum Institute of Indias Covishield and Bharat Biotech s Covaxin.
Expert panel recommends emergency use approval for Covaxin. BIG update on exam pattern November date-sheet buzz All you need to know. The WHO will take a call on granting Emergency Use Listing EUL status for Bharat Biotechs COVID-19 vaccine Covaxin next week the global health body said on Tuesday.

When Can We Expect Who Approval For Covaxin

Good News For Those Who Have Taken Covaxin Who Set To Give Nod To Bharat Biotech S Vaccine Youtube

Fact Check Covaxin Has Not Been Approved For Children Above 12 Years Yet Fact Check News

Indian Travellers Are Worried About Covaxin S Who Approval Quartz India

Who S Covaxin Clearance Delayed Further Over Technical Queries Sources

Covaxin S Approval To Be Discussed On 5 Oct

Covaxin Bbv152 For The Treatment Of Covid 19 India

Who To Decide On Bharat Biotech S Emergency Approval For Covaxin In October
India S Covaxin Yet To Receive Who Endorsement Govt To Push For Approval The Economic Times Video Et Now

As Indians Who Took Covaxin Await Approval For Travel What S Causing Delay In Who Nod
Why Was Bharat Biotech S Covaxin Not Approved In Us Here S What We Know So Far Latest News India Hindustan Times
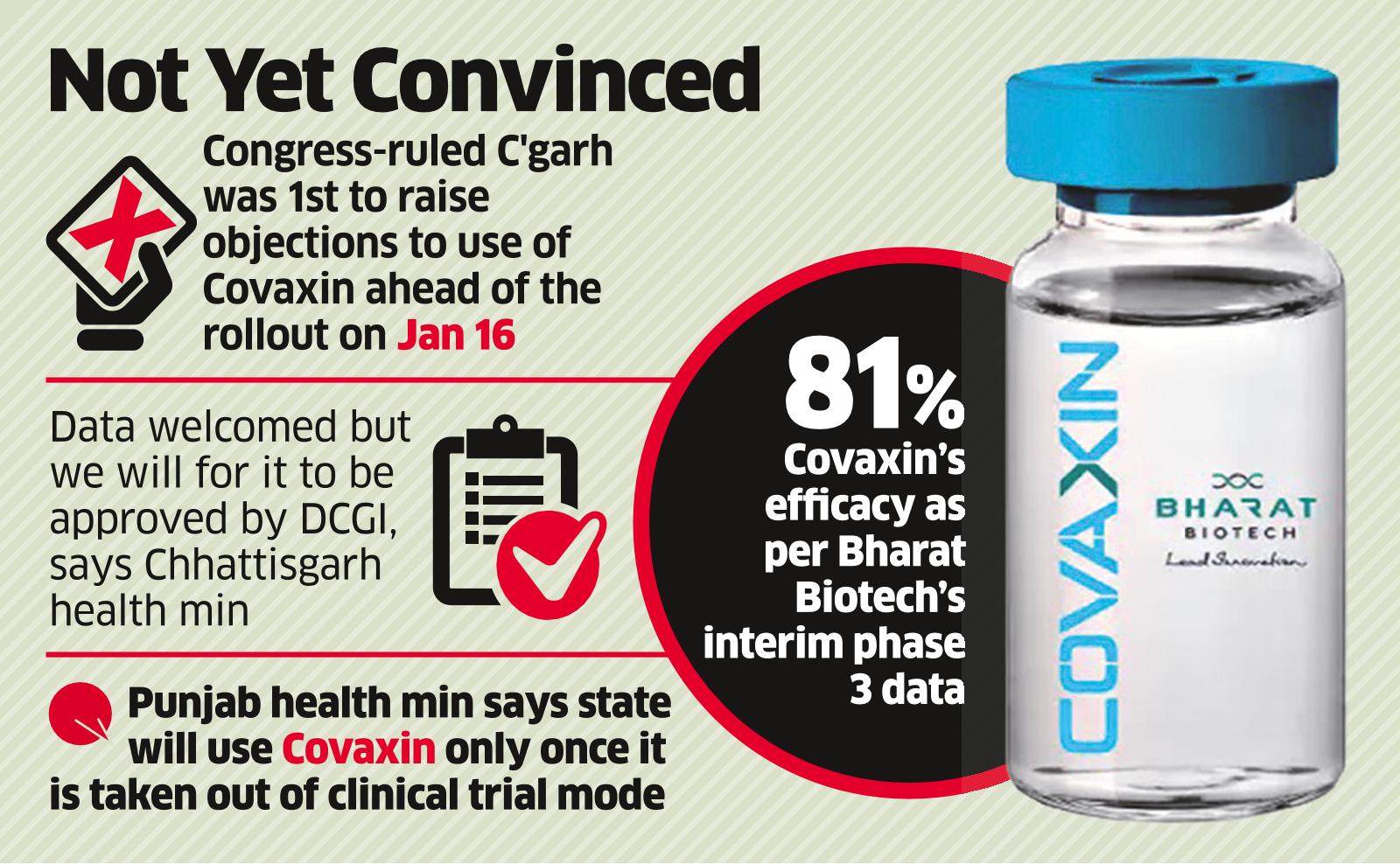
Covaxin Efficacy Data Has No Effect On Opposition States The Economic Times

Who Approval For Covaxin May Come By Month End Says Niti Aayog

Who Approval For Covaxin Likely In Mid Sept Businesstoday

Who S Approval For Covaxin Bharat Biotech S Pre Submission Meeting On June 23

Covaxin May Soon Get Who Approval As Expert Group Scheduled To Meet Next Month

India Gets 2 Vaccines Against Coronavirus As Covaxin Covishield Get Final Nod By Dcgi
Aiims Covaxin Effective For 9 12 Months No Serious Side Effects So Far Business Standard News
Coronavirus India Approves Covid 19 Vaccines Covishield And Covaxin For Emergency Use The Hindu
