Covaxin Who Approval Canada, Approval For Covaxin Sought In Canada
Read Bharat Biotech partner seeks Covaxin approval in Canada. All India Reported by Akhilesh Sharma Edited by Anindita Sanyal Updated.
Covaxin Vaccine Ocugen To Seek Emergency Nod For Covaxin From Health Canada India News Times Of India
UPDATED ON AUG 05 2021 1106 AM IST.
Covaxin who approval canada. The reason being that he has been vaccinated with Covaxin the anti-Covid-19 vaccine developed by Bharat Biotech which is yet to get approval from the World Health Organization WHO. Bharat Biotechs Covaxin is trying to seek approval in Canada. In Canada four COVID-19 vaccines have been reviewed and authorised for use by Health Canada Pfizer-BioNTech Comirnaty Moderna Spikevax AstraZeneca VaxzevriaCOVISHIELD JanssenJohnson Johnson.
The company has now approached Health Canada and submitted the Phase 3 clinical trial data of Covaxin. As a result of pending approval of WHO several countries such as the United States the United Kingdom Germany Canada etc. While the manufacturers of the Covid-19 vaccine Covaxin have completed their submission to Canadian health authorities for emergency use they have also.
The US National Institute of Health said people who had received Covaxin suggest that the vaccine generates antibodies that effectively neutralise the B117 Alpha and B1617 Delta variants. According to the authority the approved vaccines in UAE have been confirmed as follows. The World Health Organisations WHO approval for Bharat Biotechs COVID-19 vaccine Covaxin is expected this week sources said on Monday.
Bharat Biotech has submitted its Phase 3 clinical trials data that. To date these vaccines are either whole virus inactivated vaccines eg. To depart from Canadian airports or travel on VIA Rail and Rocky Mountaineer trains travellers will need to qualify as a fully vaccinated traveller.
If the vaccine gets approved for emergency use in Canada it is a sigh of relief for all those. A WHO panel is scheduled to meet this week to review Covaxins application. At present eight countries apart from India have authroised the use of Covaxin including Guyana Iran Mauritius Mexico Nepal Paraguay Philippines.
Starting October 30 2021. Bharat Biotech partner Ocugen seeks approval for Covaxin in Canada The rolling submission process was recommended and accepted under the. This will also apply to travellers on cruise ships when the cruise season commences in 2022.
Have been treating the Indian citizens vaccinated with Covaxin as un-vaccinated the plea filed through advocate Shriya Gilhotra said. Bharat Biotechs Covaxin has been reportedly placed under review for emergency-use authorization by Canada. Covaxin Another Step Closer to WHO Approval.
Covaxin under review of Canadian health regulator. Covaxin to Get Approved in Canada for Emergency Use. Know the Status Process Acceptance in Other Nations File photo of Bharat Biotechs Covaxin.
Ocugen only announced it was seeking approval in Canada on Thursday. The company has now approached Health Canada and submitted the Phase 3 clinical trial data of Covaxin. Covaxin to Get Approved in Canada for Emergency Use Bharat Biotechs Covaxin is trying to seek approval in Canada.
The company looks to get authorization from the government to sell the vaccine in the country. The application was filed at the end of June by Ocugen a Pennsylvania-based pharmaceutical company through its Canadian affiliate Vaccigen Ltd. The company looks to get authorization from the government to sell the vaccine in the country.
Pfizer-BionNTech Oxford-AstraZeneca or Covishield Sinopharm Sputnik. The authority also responded to queries on whether passengers who took the Covishield vaccine that is being administered in India would be accepted as per the new travel protocols. A number of vaccines are available in other countries that are not authorized for use in Canada.
In June Ocugen entered into an agreement with Bharat Biotech to develop manufacture and commercialise the COVID-19 vaccine Covaxin in Canada in addition to the existing rights in the United States. Notably Vaccigen Ltd is the Canadian affiliate of Ocugen-- which is Bharat Biotechs partner for the US. Places including the US Canada Australia Ireland and the European Union do not recognise Covaxin as an approved vaccine for now.
Covaxin Bharat Biotechs India-made vaccine against the coronavirus disease Covid-19 was not given approval for emergency use in the United States by the countrys top public health regulator. OCGN stock the Covaxin vaccine for the novel coronavirus will be approved by the FDA only around the. Ocugen has already approached Health Canada and submitted the Phase 3 clinical trial data of Covaxin seeking authorization to sell the jab in that country.
According to the data listed on Health Canadas official website a vaccine candidate by Vaccigen Ltd has been placed in its list of COVID-19 related treatments. Which Countries Have Approved Covaxin. However the company has submitted all the necessary documents to the Canadian government.
Covaxin has been recognised in 22 countries so far and hopefully it will be approved by WHO soon sources said. Approval from the World Health Organisation for Bharat Biotechs vaccine Covaxin is likely to come in mid-September. New Delhi India September 13 ANI.
The city-based vaccine maker recently said it concluded the final analysis of Covaxin efficacy from Phase 3 trials. Ocugen only announced it was seeking approval in Canada on Thursday. COVAXIN is considered an investigational drug in Canada and the United States and has not been approved or authorized for use in those countries.
Citing the fact that the vaccine has shown good results against the Delta variant as well. In the rosiest of scenarios for Ocugen NASDAQ. Ocugen currently holds an agreement to produce Covaxin in Canada in addition to the existing rights in the United States.
Health Canada is considering approving Covaxin a COVID vaccine developed in India after quietly accepting an application from its American distributor. The World Health Organization WHO is likely to grant emergency use approval to Bharat Biotechs Covaxin by mid-September. A WHO panel will meet.

Gujarat Students Canada Dreams And Covaxin Conundrum Ahmedabad News Times Of India
Bharat Biotech S Partner Ocugen Seeks Approval For Covaxin In Canada The Hindu Businessline
India S Covaxin Vaccine Shows High Efficacy Against Covid 19 Infections In Phase 3 Trial Gavi The Vaccine Alliance
Bharat Biotech Ocugen Pays 15 Mn Upfront To Bharat Biotech For Covaxin Rights In Canada The Economic Times

Bharat Biotech S Partner Ocugen Seeks Approval For Covaxin In Canada The Economic Times
Covid 19 Ocugen To Seek Emergency Use Approval For Covaxin In Canada Latest News India Hindustan Times
Bharat Biotech S Partner Ocugen Seeks Approval For Covaxin In Canada The Hindu Businessline
Ocugen Pays 15 Million Upfront To Bharat Biotech For Covaxin Rights In Canada The Hindu

Ocugen Preps Filings For Covid 19 Vaccine Covaxin On New Data

Bharat Biotech S Partner Ocugen Seeks Approval For Covaxin In Canada The Financial Express
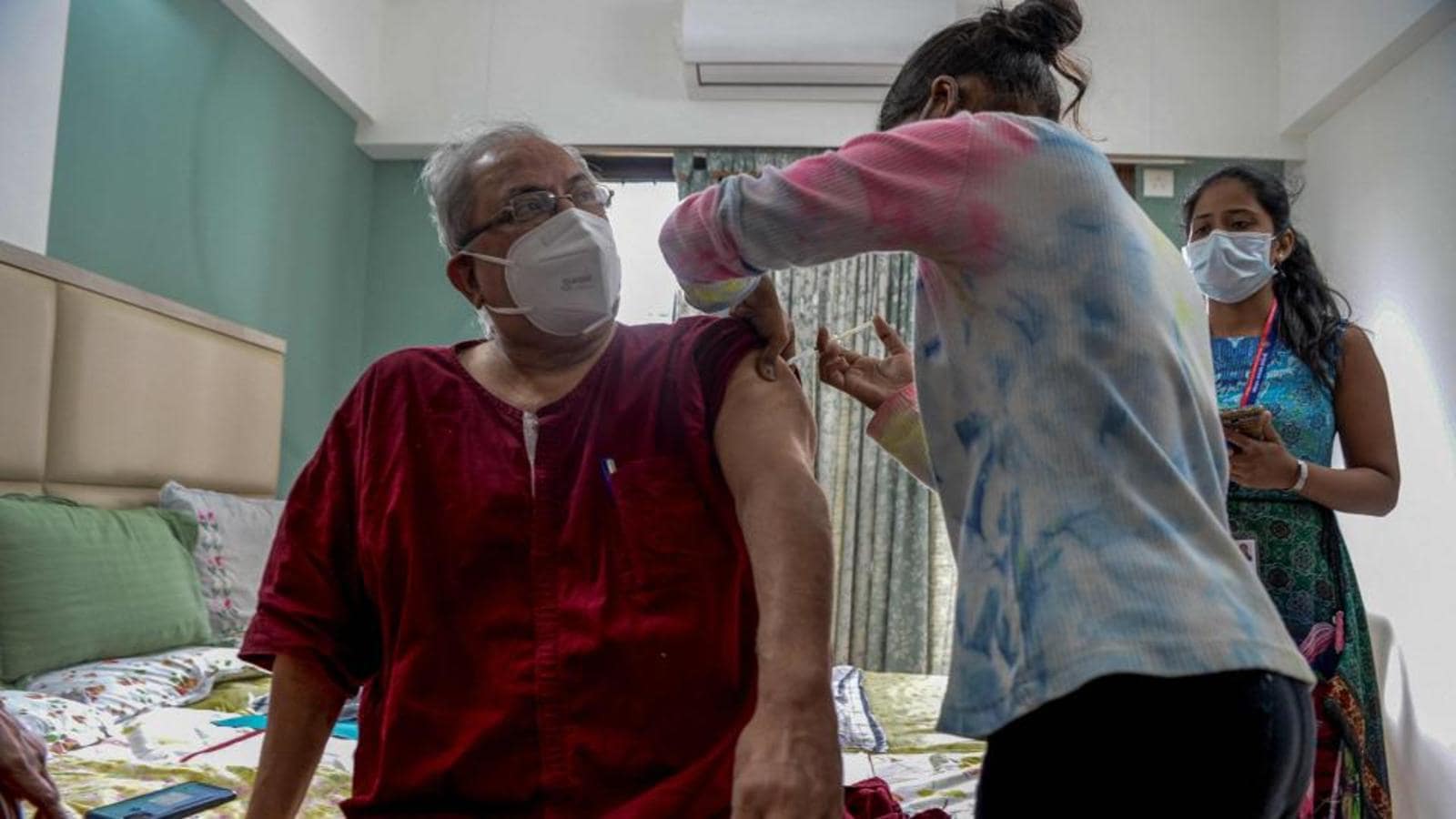
Covid 19 Covaxin Submits Documents For Emergency Use Authorisation In Canada World News Hindustan Times

Gujarat Students Canada Dreams And Covaxin Conundrum Ahmedabad News Times Of India
Zydus Cadila What We Know About India S New Covid Vaccines Bbc News

Approval For Covaxin Sought In Canada

Faq I Have Taken Covaxin Will I Be Allowed To Travel Abroad

Canada To Review Bharat Biotech S Covaxin Covid Vaccine For Emergency Use Authorization

Fda Denies Bharat Bio S Us Partner Emergency Approval For Covaxin Vaccine Business Standard News
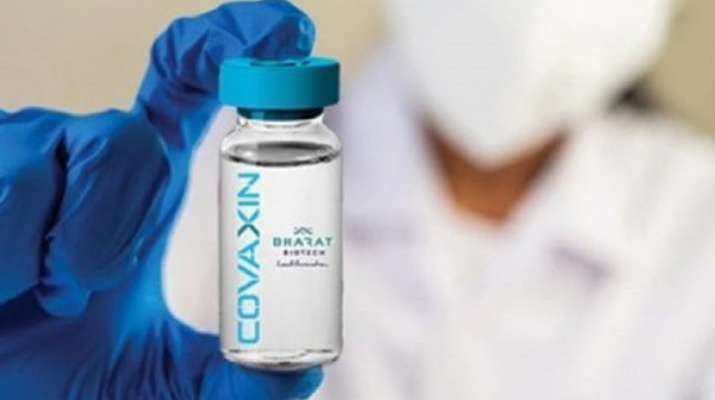
Bharat Biotech Partner Ocugen Seeks Approval For Covaxin In Canada India News India Tv

Good News For Those Who Have Taken Covaxin Who Set To Give Nod To Bharat Biotech S Vaccine Youtube
